this post was submitted on 12 Feb 2025
709 points (99.2% liked)
Science Memes
14653 readers
3596 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
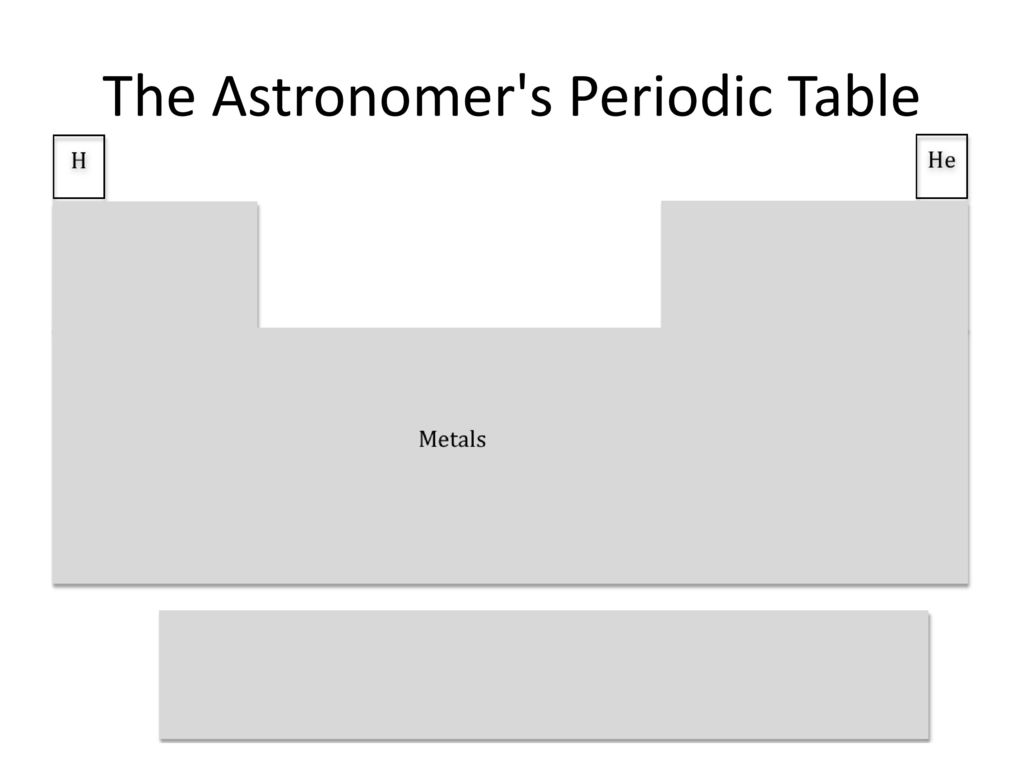
The intended joke is that hypervalent iodine compounds like Dess-Martin periodinane flip between different oxidation states like you often see for transition metals. As an example, the mechanism usually drawn for oxidations by DMP is similar to those drawn for PCC/Jones reagent, where the electrons removed from the substrate are "banked" at the metal center. Obviously, redox chemistry is not at all limited to transition metals, but I am often surprised at iodine's propensity to engage in it. A lot of research over the past decade or two has also developed redox catalysis with these reagents, reactivity which is commonly (though again not always) the purview of transition metals.