this post was submitted on 22 Apr 2024
2 points (100.0% liked)
Science Memes
10853 readers
3531 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
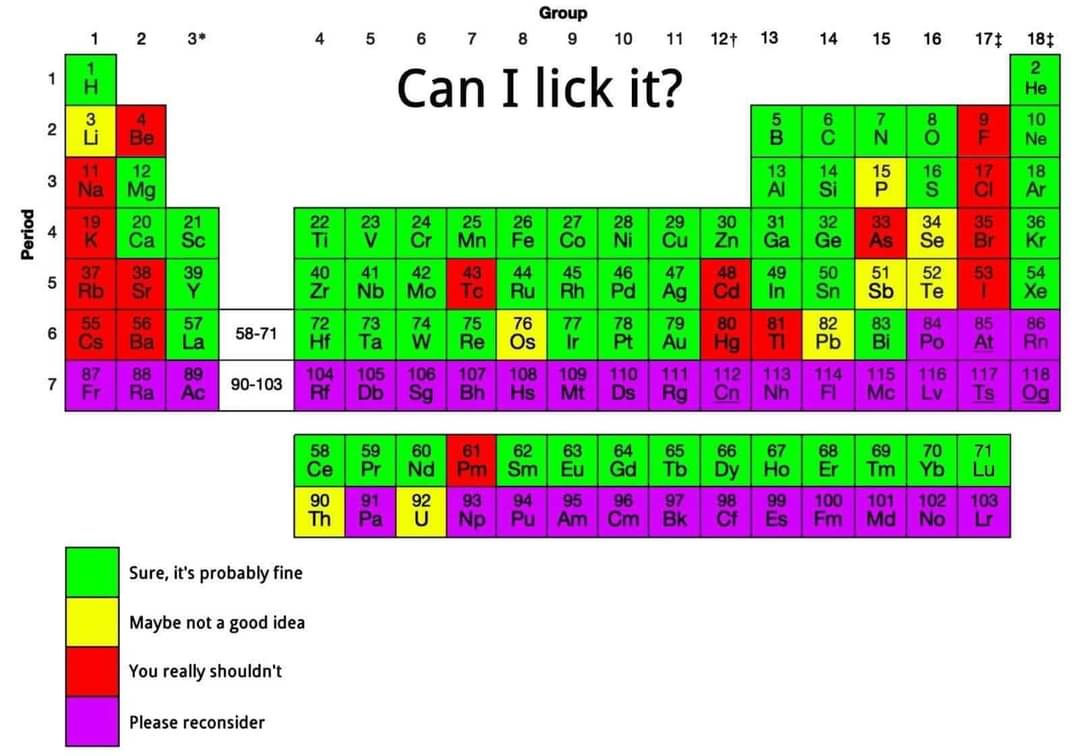
This is the part I don't understand. If charge is conserved, why would there be a preference for a particular charge in the products?
No. I think that you're absolutely correct. The products should have charge conserved. After initial attack of hydrocarbons by H• radicals, H2 is likely to be a significant product. Supposing STP, it would likely remove itself from solution, leaving the fresh radicals to chain react and probably making interesting and unhealthy things.
My apologies, I'm out of the lab and field 15 years now so, do make some pretty basic mistakes at times.
That makes sense.
No worries! I've enjoyed this discussion!